
Experimental and theoretical constraints on the MgO phase diagram are summarized in Figure 14.
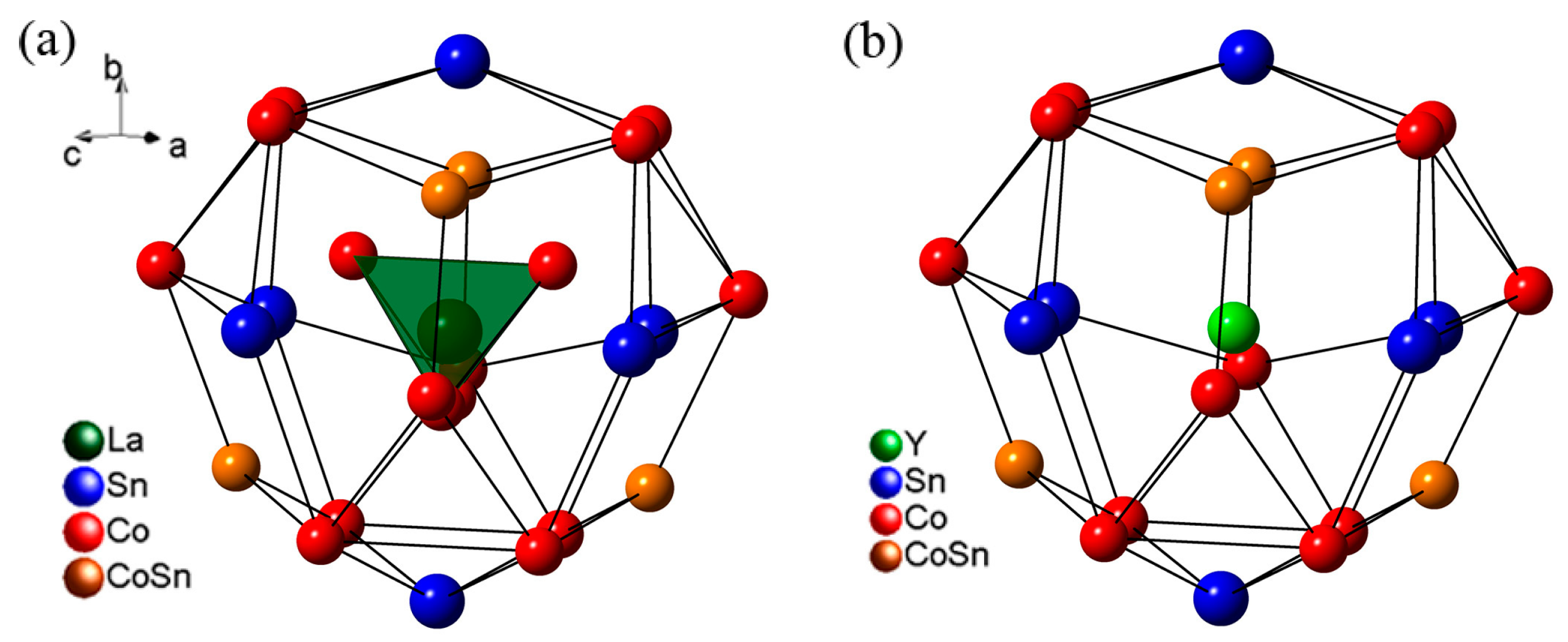
MgO crystallizes in the B1 rock-salt structure, and static compression experiments have shown that the B1 phase remains stable to at least 250 GPa ( Dorfman et al., 2012 Duffy et al., 1995). Its high-pressure behavior has attracted much attention due to its simple structure, wide stability field, and geophysical importance. MgO ( periclase) is the end-member of the (Mg,Fe)O solid solution. Inclusions containing periclase derived from the solar nebula have been found in chondritic meteorites.
1C) have been offered for sale under the pretense that they are natural ( Peretti et al., 2018). Where it is altered it usually produces brucite (Mg(OH) 2).ĭue to the mode of formation, natural periclase always occurs as grains that are too small for the gem market larger synthetic crystals ( Fig. The temperatures required to form periclase depend upon both pressure and the availability of CO 2 as demonstrated by Cook and Bowman (1994) and illustrated in Fig. Periclase is a relatively high temperature mineral that occurs, for instance in marbles that result from the metamorphism of dolomites and magnesian limestones. Small amounts of Cr and Fe produce green periclase and Mn-Zn-bearing periclase is grass-green in color ( Fig. The color of periclase varies from white to yellow, or brown when iron is present. Periclase derived from heating of dolomite is useful as a refractory material, for instance as a lining to blast furnaces. Contributions to Gemology, Special Alert Issue, 1–31. (C) Reproduced with permission from Peretti A, Bieri W, Cleveland E, Alessandri M, Kradolfer S, Gunther D, Meier M, Jaramillo D, and Musa M (2018) New Types of Synthetic Periclase Identified by C 2 S Inclusions- Dicalcium Silicate Cement Hardener. (B) Reproduced with permission from Bowles JFW, Howie RA, Vaughan DJ and Zussman J (2011) The Rock Forming Minerals, vol.
#REPEATING A UNIT CELL IN CRYSTALMAKER SOFTWARE#
Oxford, England: CrystalMaker Software Ltd.
#REPEATING A UNIT CELL IN CRYSTALMAKER FOR MAC#
(A) Crystal/Maker®, Crystal Structure Images Generated Using CrystalMaker®: A Crystal and Molecular Structures Program for Mac and Windows. The stability fields of periclase, calcite, brucite and dolomite are labeled for the experimental run at 100 MPa.
(B) T–X CO 2 phase equilibria for periclase–brucite–calcite–dolomite at a constant lithostatic pressure of 150 MPa and fluid pressures (P f) of 50, 75, 100 and 150 MPa.


 0 kommentar(er)
0 kommentar(er)
